Peru (a hospital in Iquitos shown here) has been one of the nations hit hardest by the COVID-19 pandemic.Credit: EFE News Agency/Alamy
As much of the world waits for an effective vaccine to curb the COVID-19 pandemic, some in Latin America are turning to an unproven treatment. There isn’t enough evidence that the drug, ivermectin, is safe or effective as a coronavirus therapy, however. So researchers are cautioning against using it outside clinical trials. Still, people in the region have rushed to take it, making it hard for researchers to properly test it.
Ivermectin, an inexpensive, over-the-counter medicine, has been used for decades to treat livestock and people infested with parasitic worms — and in the past few months, its popularity as a preventative against COVID-19 has surged in Peru, Bolivia, Guatemala and other Latin American countries.
The drug has been so in demand that in May, health-care workers passed out some 350,000 doses to residents in northern Bolivia. That same month, the Peruvian police seized around 20,000 bottles of animal-grade ivermectin that was sold on the black market as a treatment for human coronavirus infections. And in July, a university in Peru announced that it would produce 30,000 doses to bolster the country’s supply.
But the evidence that ivermectin protects people from COVID-19 is scant. Some early studies in cells and humans hinted that the drug has antiviral properties, but since then, clinical trials in Latin America have struggled to recruit participants because so many are already taking it. ...
Peru (a hospital in Iquitos shown here) has been one of the nations hit hardest by the COVID-19 pandemic.Credit: EFE News Agency/Alamy
As much of the world waits for an effective vaccine to curb the COVID-19 pandemic, some in Latin America are turning to an unproven treatment. There isn’t enough evidence that the drug, ivermectin, is safe or effective as a coronavirus therapy, however. So researchers are cautioning against using it outside clinical trials. Still, people in the region have rushed to take it, making it hard for researchers to properly test it.
Ivermectin, an inexpensive, over-the-counter medicine, has been used for decades to treat livestock and people infested with parasitic worms — and in the past few months, its popularity as a preventative against COVID-19 has surged in Peru, Bolivia, Guatemala and other Latin American countries.
The drug has been so in demand that in May, health-care workers passed out some 350,000 doses to residents in northern Bolivia. That same month, the Peruvian police seized around 20,000 bottles of animal-grade ivermectin that was sold on the black market as a treatment for human coronavirus infections. And in July, a university in Peru announced that it would produce 30,000 doses to bolster the country’s supply.
But the evidence that ivermectin protects people from COVID-19 is scant. Some early studies in cells and humans hinted that the drug has antiviral properties, but since then, clinical trials in Latin America have struggled to recruit participants because so many are already taking it. ...
Ivermectin grabbed attention in April, when scientists were throwing every already-approved drug they could at the coronavirus. Researchers in Australia had noted that high doses of ivermectin could stop the virus from replicating in cells1. Shortly afterwards, a preprint appeared online that suggested the drug could reduce coronavirus-related deaths in people.
That report was later removed from the site by some of its authors because, they told Nature, the study was not ready for peer review. The preprint had included an analysis of electronic health records by the company Surgisphere, which provided unreliable COVID-19 data sets that raised red flags for scientists in late May. By June, two other high-profile COVID-19 studies were retracted that contained data from the firm. ...

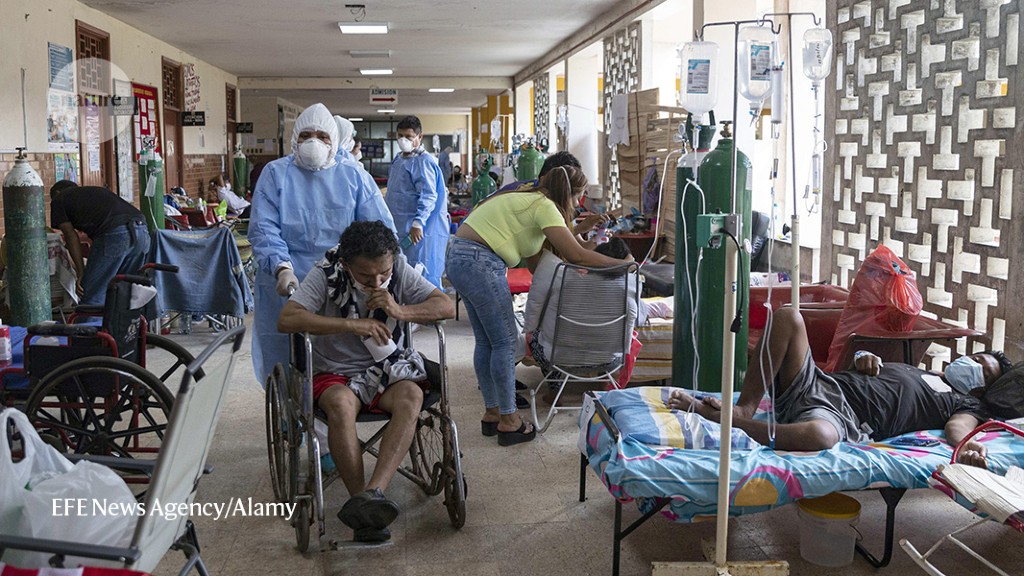

Recent Comments